Stay Compliant
As the medical industry’s needs for mass processing and management of data continues to soar, the need for scalable and fast-delivery solutions continues to rise in turn. This demand, coupled with continual efforts to reduce costs has naturally led medical industry providers to seek out cost-effective solutions that minimize the IT management burden and maximize capacity for data and growth. SaaS solutions are a perfect fit for this need but concerns about compliance and security must be addressed, particularly when it comes to HIPAA and FDA regulations.
What do regulators care about?
It’s about fitness for purpose, not an inventory of IT equipment
Regulators are not averse to cloud solutions, they simply need to understand the risks and required controls. As SaaS solutions in the life sciences industry are now common, regulatory agencies are adapting their GxP assessments to fit the technology landscape.
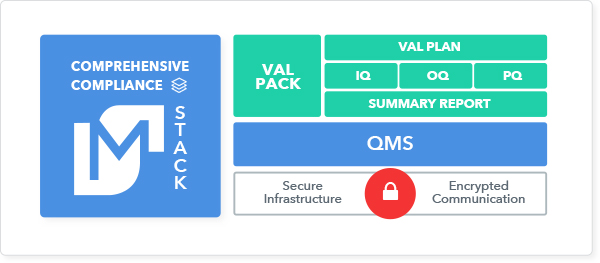
With a comprehensive compliance framework, regulators have a complete picture of the intended purpose of the solution and how it is controlled. To fulfill both FDA and HIPAA requirements, the framework must include a thorough validation package, a QMS with controls across the organization, and a secure application infrastructure that encrypts PHI communications.
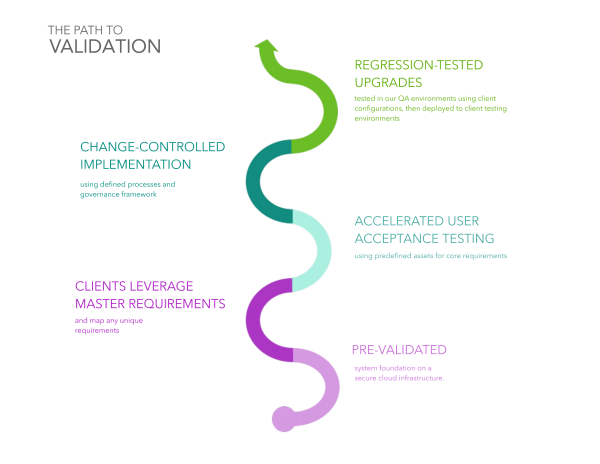
How is FDA validation achieved in a SaaS model?
With simple-to-use tools and highly-repeatable processes
Maintaining the validated state of a SaaS solution requires a repeatable process that minimizes the burden of high-volume tasks such as authoring requirements and conducting regression testing.
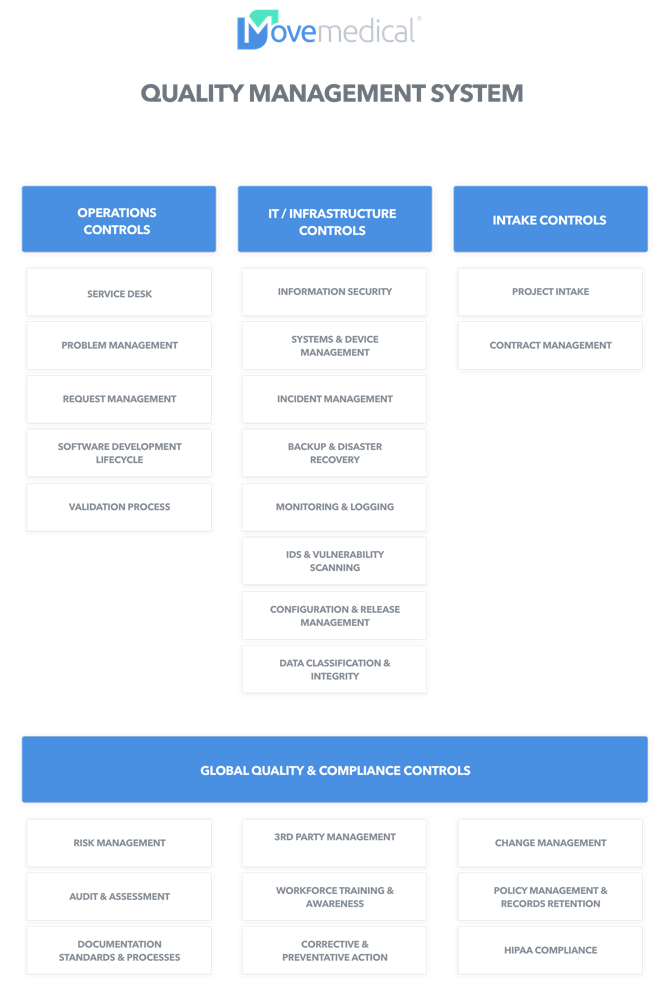
What’s in a good Quality Management System?
Coverage at every level.
A comprehensive Quality Management System addresses not only the basic IT needs, but a broad set of controls across all domains
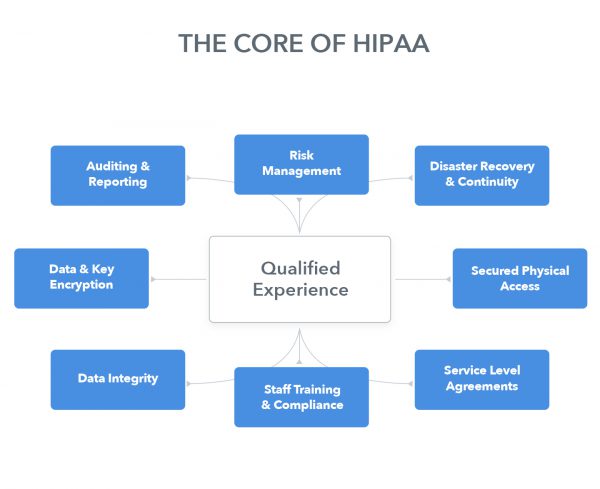
What about HIPAA? How does SaaS address it?
Inter-networked controls backed by deep industry experience.
There is no specific provision in the HIPAA guidelines that opposes use of a cloud application. However, HIPAA guidelines pertaining to encryption, user authentication and other best practices still must be satisfied. Medical entities need a solution that meets and exceeds the Administrative, Technical, and Physical Safeguards established in HIPAA Security Rule.
To demonstrate HIPAA compliance, a solution provider should be able to address the key safeguards prescribed by HIPAA. However, without qualified experience, controls tend to serve as disconnected, stifling, and ineffective measures that miss the mark. A provider must possess relevant experience to truly understand the operations and challenges of the industry.
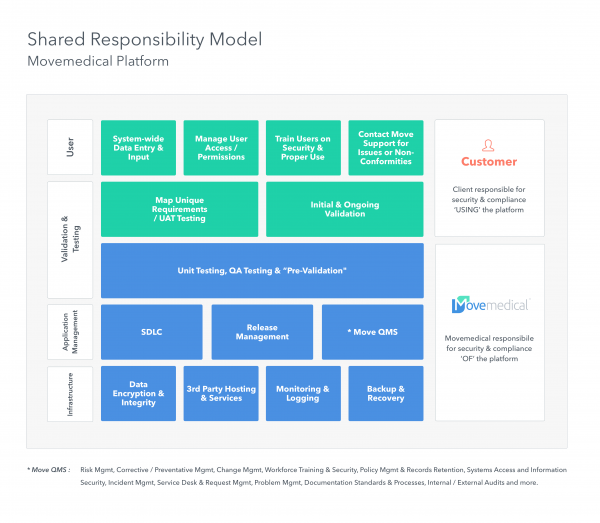
What am I responsible for?
With a good software provider, far less than you are used to.
Inherently, a SaaS provider assumes responsibility for much of the IT and compliance burden. Ultimately clients own compliance of their instance of a solution, but the majority of the requirements can be streamlined if the provider has addressed regulations in a thorough manner.
This model illustrates the optimal approach to responsibility within a SaaS platform.
Summary
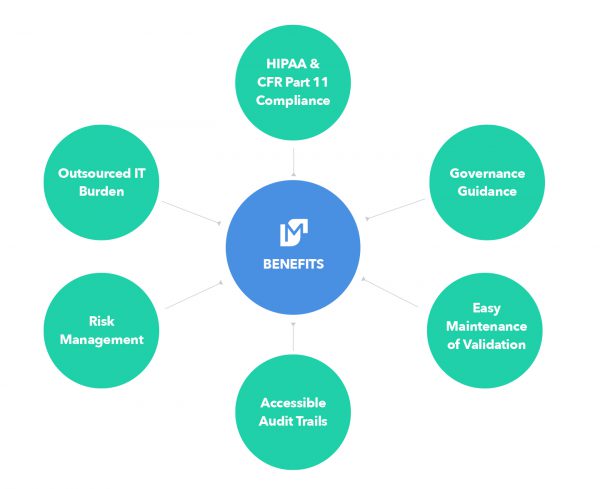
Quality management and regulatory compliance is often an afterthought of a software solution, and for a SaaS solution a challenging one at that. In contrast, Movemedical has made the Quality Management System and regulatory requirements the foundation of the solution. This translates into simpler maintenance of the application and its controlled state.
Movemedical leverages extremely powerful and scalable state-of-the-art cloud services while enabling that GxP compliance. Movemedical performs all development and infrastructure design of its inventory management solution. Software development activities utilize a 21 CFR Part 11, CFR Part 820.70(i) compliant Systems Development Lifecycle.
Note: Movemedical provides a host of functionality. Classification of the Movemedical platform as a GxP or healthcare-regulated solution will vary by customer based on their intended use. Additionally, the Movemedical platform technically cannot be considered a validated solution since validation must be performed against a given customer’s implementation. To help customers achieve validation, Movemedical offers validation services or assets to facilitate a customer’s validation of their implementation.
Disclaimer: Movemedical is not a legal advisor. No part of this content is intended to be, or should be construed as legal advice or opinion. Please contact an attorney for legal counsel.
Contact us: 858.956.0219 or info@movemedical.com
WATCH DEMO NOW (you can personalize it to your needs/wants)
A good field inventory solution and/or a WMS are worth their weight in gold—but a fully integrated medical device-specific operations and sales force effectiveness platform is priceless.
A complete tool should be able to or have:
- Surgery Scheduling (+ Calendar)
- CRM / Case Management
- Directed Picking & Put-away (Bin Mapped)
- Active Order Visibility
- Shipping Coordination (FedEx, UPS Integration)
- Customer/Account Management
- Opportunity/Lead Management
- Surgeon Preferences / Preference Cards
- Inventory Control & Visibility (All Locations, All Buckets)
- Auto Product Replenishment
- Commission Calculations
- Expiration Notifications
- Direct Orders
- Send & Receive Tools (Rep to Rep etc.)
- Contract & List Pricing (Real-time)
- Audit/Cycle Counts
- Reporting & Billing
- Image & File Sharing
- Secure HIPAA Compliant Communication
- Mobile Usage Capture
- UDI Compliance
- Barcode Scanning
- RFID Integration & RFID Reconciling (HF/UHF)
- ERP/CRM/EMR Integrations
- Demand Planning Tools
- Consignment/Loaner Management
- Pluggable Workflows (Programmatic Integrations, Integrated Prediction Models)
- Sourcing Matrix Tool
- HIPAA Compliant Communication Platform (Messages, Calendar, Usage, Notes, Files)
- Sourcing Optimization
- Future Stock (Virtual Inventory Assigned to Future Events)
- Atomic Inventory (UDI Ready, Piece Level Tracking, Serialized or Not)
- Hybrid Kitting (Skinny Kits, Kit Management, Kit Versioning, Tracking)
- Cross Boundary Workflows
- Lost & Found (Automatic Cycle Counting )
- Merger/Acquisition Integration
- Par Management
- Multi-Catalog Management
- Loan Optimization (1 Loan Per Day)
- Separate or Combined Sales & Operations Alignment
- Cost-to-Serve Metrics
- Turn Ratio Dashboards
- Sales Metrics / Data (Reps, Leaders, Corporate)
Need to talk with someone? We understand. Call anytime.
phone: 858.956.0208
email: mm@movemedical.com
or visit: www.movemedical.com/product