Q&A with the Operations Supervisor of a Large Independent Orthopedic Implantable Device Distributor “[T]he service we provide to our customers is becoming more proactive instead of reactive. It’s a good thing!” Problem Tami B., has been an Operations Supervisor for one of the world’s largest orthopedic companies for over 20 years. For Tami, getting both the operations teams and the sales force to securely and effectively communicate business needs has always been a challenge. Reps
Case Study: How This Vice President of Operations Reduced Field Inventory & Increased Turns
Category: Case Study
Frank* is a Vice President of Operations that has worked in the orthopedic medical device industry for over 25 years. The Problem: Major Organizational Challenges Outdated Processes: All scheduling and sourcing was done manually, via paper case requests and paper schedules. Disconnected Systems: The software that managed the resources ran independently of the billing and replenishment processes. Poor Visibility: Inventory management and schedule suffered do to lack of visibility and accountability. Frustrations & Pain Points
It’s War Out There. Should It Be?
Category: Article
The Ongoing Battle Will the right parts be there on time? Will they be the correct implants and instruments? How much time do I spend checking, correcting, and tracking down inventory? Did the rep really mean to order what he just requested? These are some of the questions sales and ops folks ask themselves everyday. In the medical device space the battle has always been Operations vs. Sales. The ongoing war usually means that there
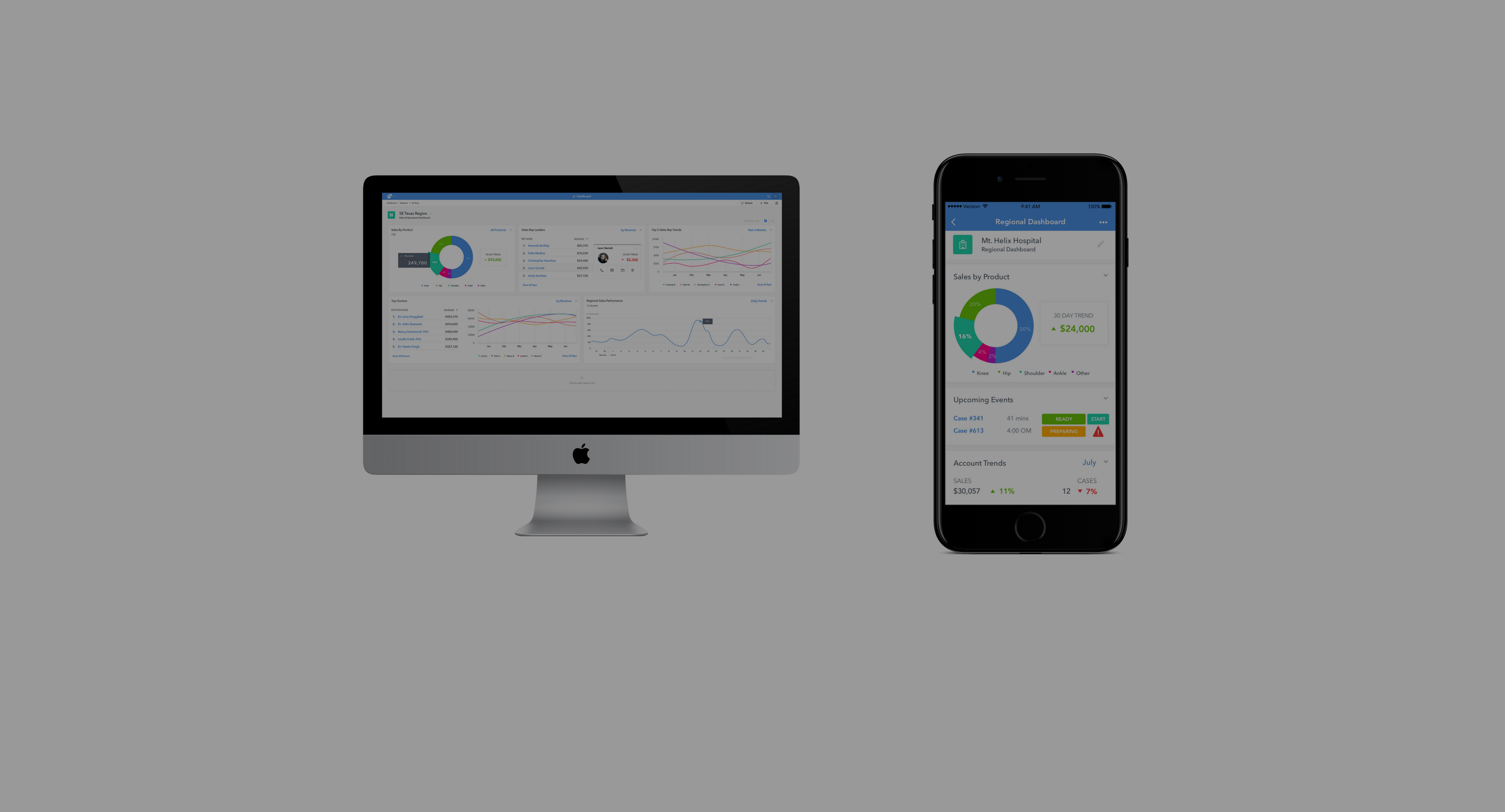
GIVE YOUR TEAM THE FULL INVENTORY CONTROL IT DESERVES Your organization is constantly battling inventory traceability, lot control, demanding schedules, last-minute requests, complexity, and, as always, costs. Movemedical is here to help. When you have the ability to track each piece of stock individually you are empowered with complete visibility of every single item along with loan shipping optimization, audit management, stock location predictions, auto-reconciliation, and the holy grail of medical logistics: future
FDA Unique Device Identification (UDI) requirements are coming. The challenges they bring are substantial, and solutions are varied and often complex. Although the medical device supply chain is filled with obstacles for manufacturers, distributors, and hospitals UDI is another step on the path to progress. As the new UDI requirements are implemented, visibility will be gained. Healthcare supply chains, including medical device manufacturers and hospitals, must work together to increase visibility for everyone. Separate Tools